BioLineRx Reports Second Quarter 2013 Results
JERUSALEM – August 6, 2013 - BioLineRx Ltd. (NASDAQ: BLRX; TASE: BLRX), a biopharmaceutical development company, today reported its results for the quarter ended June 30, 2013.
Recent Highlights:
- BL-1040 (ventricular remodeling) – The PRESERVATION I clinical trial, a CE-Mark registration trial, is proceeding as scheduled – there are currently 36 active sites in 9 countries, including 7 leading sites in the U.S.
- BL-5010 (skin lesions) – The necessary regulatory submissions to commence a pivotal, CE-Mark registration trial in Europe have been filed; the CRO and three sites in Germany have been selected.
- BL-8040 (AML) – The ongoing Phase 2 clinical trial is progressing as planned, and currently has 6 active sites recruiting (out of 8 sites in total); the first patient was enrolled in the study at the MD Anderson Cancer Center in Houston; Northwestern Memorial Hospital in Chicago was recently initiated into the trial and is expected to commence recruitment in the coming weeks; enrollment has been initiated at several leading sites in Israel following approval from the Israeli Ministry of Health;
- BL-7010 (celiac disease) - Advanced GLP toxicology studies have begun and the CRO and sites in Europe have been selected for the upcoming Phase 1/2 clinical trial, expected to commence in Q4 2013; results of pre-clinical studies were presented and received with enthusiasm at two leading international meetings - the U.S. Celiac Disease Foundation’s annual conference and the Digestive Disease Week conference.
Commenting on the ongoing progress within the Company’s pipeline, Kinneret Savitsky, Ph.D., Chief Executive Officer of BioLineRx, remarked, “The second quarter of 2013 has been a period of meaningful achievement for BioLineRx, in which several of our advanced products met important scientific and clinical milestones. Over the next few quarters, we will move forward on multiple exciting near-term opportunities across a number of therapeutic areas with significant unmet medical needs."
“We are eagerly awaiting completion of the CE-Mark registration trial for BL-1040 by our partner, Ikaria, which is expected to occur in 2014. We have high hopes that this unique product will become a breakthrough treatment for AMI and part of the standard of care,” stated Dr. Savitsky. "For BL-5010, the CE-Mark registration trial is now slated to begin enrollment later this year, setting a clear pathway for entering the European market by the second half of 2014. The estimated worldwide market for the treatment of conditions such as seborrheic and actinic keratosis is over $500 million worldwide. We estimate that BL-5010’s ease of use and low cost will allow for swift commercial acceptance, and are currently engaged in discussions with potential partners.”
“We are also very pleased with the progress of the Phase 2 clinical trial for BL-8040, a best-in-class CXCR4 antagonist for the treatment of hematological cancers such as AML. We are on track to deliver partial results from the trial in the fourth quarter of 2013, with final results expected to be available by the second half of 2014."
“Another exciting opportunity for us is BL-7010, a unique product for celiac disease, a market that is projected to reach $8 billion by 2019. Despite the huge size of this market, there are no pharmacological agents approved for the treatment of celiac disease and the only treatment option is a lifelong gluten-free diet, which is extremely difficult to maintain. There are currently only four clinical-stage projects in the world in the celiac disease development pipeline, one of which is BL-7010. We expect to initiate a Phase 1/2 safety study by the end of 2013 – we have already selected the CRO and treatment sites for the study, and are in the process of preparing the necessary regulatory submissions,” concluded Dr. Savitsky.
Financial Results:
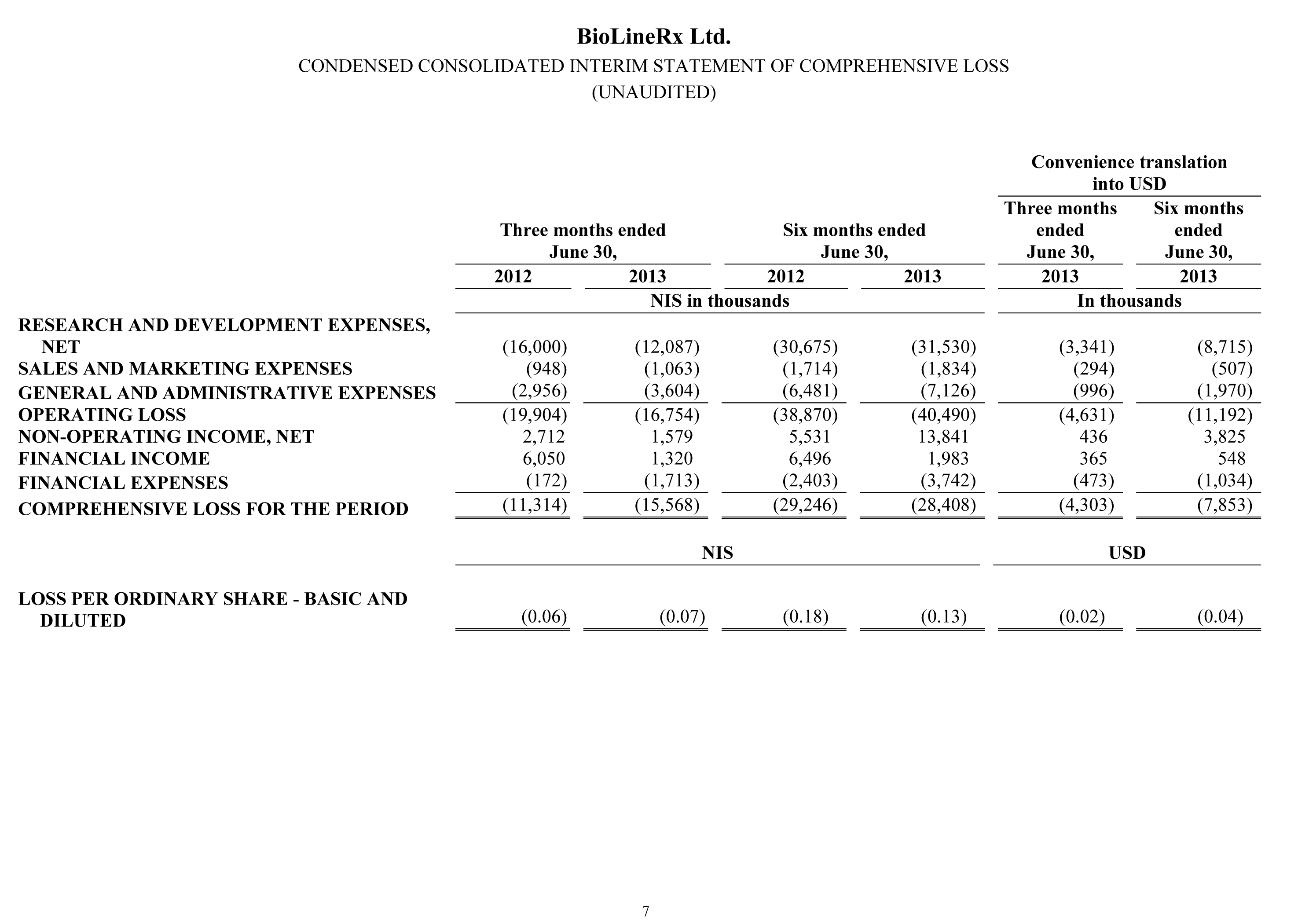
During the three-month and six-month periods ended June 30, 2013 and 2012, no revenues were recorded.
Research and development expenses for the three months ended June 30, 2013 were NIS 12.1 million ($3.3 million), a decrease of NIS 3.9 million ($1.1 million), or 24%, compared to NIS 16.0 million ($4.4 million) for the three months ended June 30, 2012. The decrease resulted primarily from lower expenses in 2013 associated with the CLARITY clinical trial in respect of BL-1020, due to termination of the trial in March 2013, offset by a ramp-up in spending on other clinical-stage projects introduced during 2012. Research and development expenses for the six months ended June 30, 2013 were NIS 31.5 million ($8.7 million), an increase of NIS 0.9 million ($0.2 million), or 3%, compared to NIS 30.7 million ($8.5 million) for the comparable 2012 period. Without regard to the NIS 6.0 million one-time reversal of amounts previously accrued to the OCS in respect of BL-1020, research and development expenses increased by NIS 6.8 million ($1.9 million). The increase resulted primarily from significantly higher expenses in the 2013 period associated with the CLARITY clinical trial, as well as a ramp-up in spending on other clinical-stage projects introduced during 2012.
Sales and marketing expenses for the three months ended June 30, 2013 were NIS 1.1 million ($0.3 million), an insignificant increase compared to NIS 0.9 million ($0.3 million) for the three months ended June 30, 2012. The small increase relates to professional fees and other expenses stemming from an increase in our business development efforts, compared to the second quarter of last year. Sales and marketing expenses for the six months ended June 30, 2013 were NIS 1.8 million ($0.5 million), an insignificant increase compared to NIS 1.7 million ($0.5 million) for the comparable period in 2012. The reason for the increase is similar to the one discussed above in the three-month comparison.
General and administrative expenses for the three months ended June 30, 2013 were NIS 3.6 million ($1.0 million), an increase of NIS 0.6 million ($0.2 million), or 22%, compared to NIS 3.0 million ($0.8 million) for the three months ended June 30, 2012. The increase resulted primarily from a one-time expense for professional services incurred in the 2013 period. General and administrative expenses for the six months ended June 30, 2013 were NIS 7.1 million ($2.0 million), an increase of NIS 0.6 million ($0.2 million), or 10%, compared to NIS 6.5 million ($1.8 million) for the comparable 2012 period. The reason for the increase is similar to the one discussed above in the three-month comparison.
The Company’s operating loss for the three months ended June 30, 2013 amounted to NIS 16.8 million ($4.6 million), compared with an operating loss of NIS 19.9 million ($5.5 million) for the comparable period in 2012. The Company’s operating loss for the six months ended June 30, 2013 amounted to NIS 40.5 million ($11.2 million), compared with an operating loss of NIS 38.9 million ($10.7 million) for the comparable period in 2012.
The Company’s net non-operating income amounted to NIS 1.6 million ($0.4 million) for the three months ended June 30, 2013, a decrease of NIS 1.1 million ($0.3 million), compared to NIS 2.7 million ($0.8 million) for the three months ended June 30, 2012. Non-operating income for both periods primarily relates to fair-value adjustments of liabilities on account of the warrants issued in the private and direct placements conducted in February 2012 and 2013. Net non-operating income amounted to NIS 13.8 million ($3.8 million) for the six months ended June 30, 2013, an increase of NIS 8.3 million ($2.3 million), compared to net non-operating income of NIS 5.5 million ($1.5 million) for the comparable 2012 period. The reason for the increase is similar to the one discussed above in the three-month comparison.
The Company recorded net financial expense of NIS 0.4 million ($0.1 million) for the three months ended June 30, 2013, a change of NIS 6.3 million ($1.7 million), compared to net financial income of NIS 5.9 million ($1.6 million) for the three months ended June 30, 2012. Net financial income and expense result primarily from changes in the average exchange rate of the dollar in relation to the NIS during the respective periods, which have a direct effect on the Company’s net assets denominated in dollars. Net financial expense amounted to NIS 1.8 million ($0.5 million) for the six months ended June 30, 2013, a change of NIS 5.9 million ($1.6 million), compared to net financial income of NIS 4.1 million ($1.1 million) for the comparable 2012 period. The reason for the increase is similar to the one discussed above in the three-month comparison.
The Company’s net loss for the three months ended June 30, 2013 amounted to NIS 15.6 million ($4.3 million), compared with a net loss of NIS 11.3 million ($3.1 million) for the comparable period in 2012. The Company’s net loss for the six months ended June 30, 2013 amounted to NIS 28.4 million ($7.9 million), compared with a net loss of NIS 29.2 million ($8.1 million) for the comparable period in 2012.
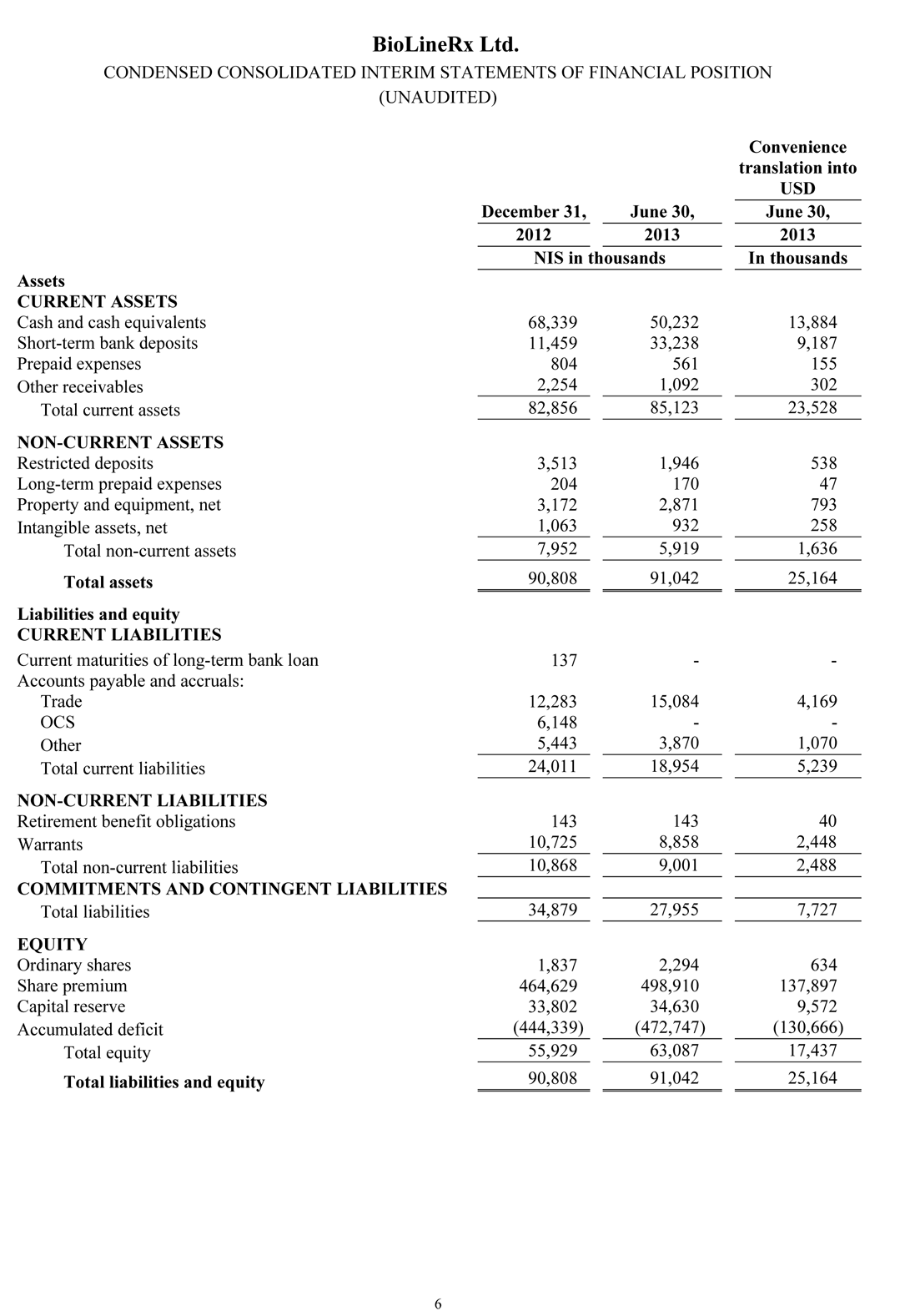
The Company held NIS 83.5 million ($23.1 million) in cash, cash equivalents and short-term bank deposits as of June 30, 2013.
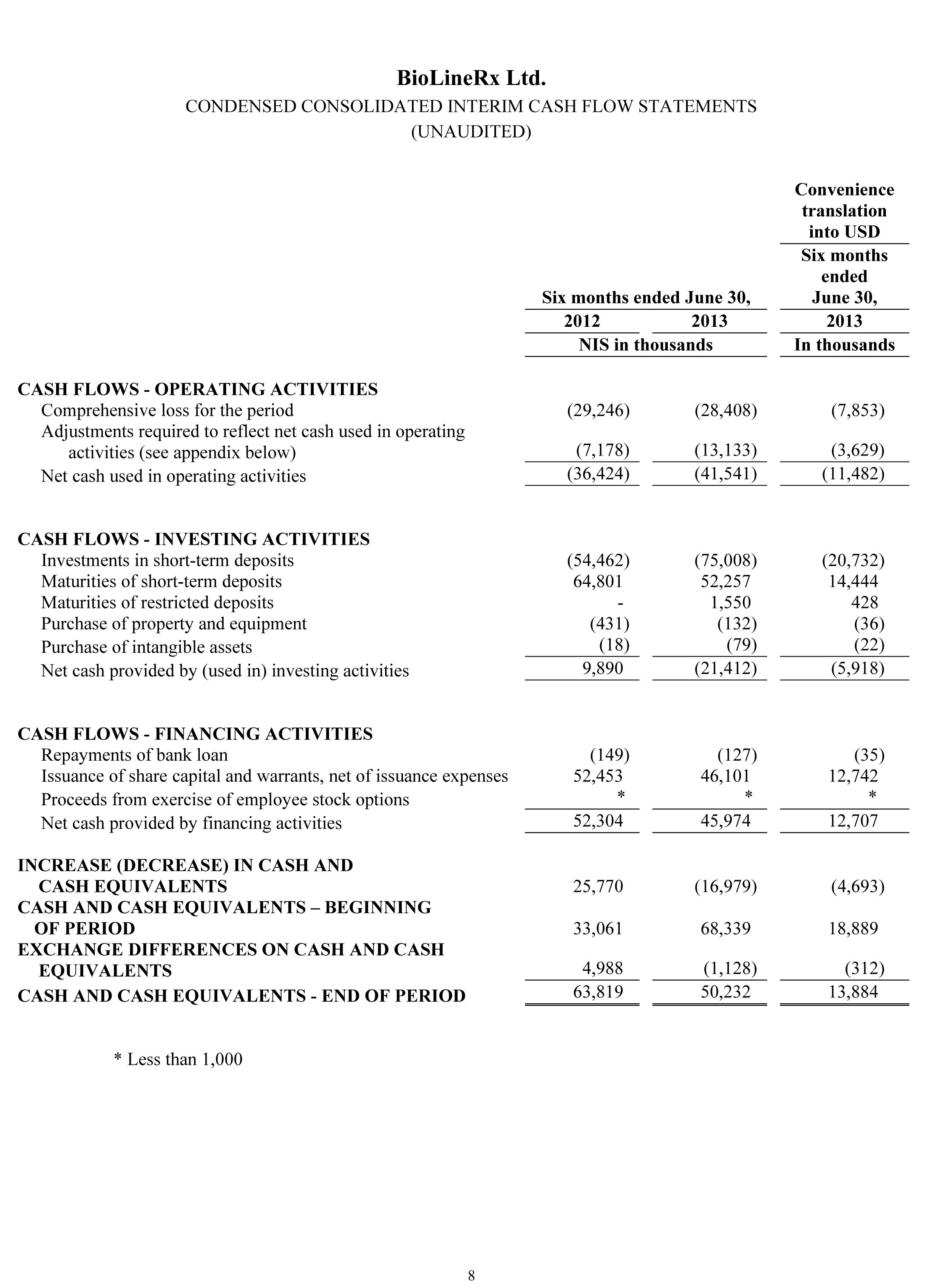
Net cash used in operating activities was NIS 41.5 million ($11.5 million) for the six months ended June 30, 2013, compared with net cash used in operating activities of NIS 36.4 million ($10.1 million) for the six months ended June 30, 2012. The NIS 5.1 million ($1.4 million) increase in net cash used in operating activities during the six-month period in 2013, versus the 2012 period, was primarily the result of increased research and development spending.
Net cash used in investing activities for the six months ended June 30, 2013 was NIS 21.4 million ($5.9 million), compared to net cash provided by investing activities of NIS 9.9 million ($2.7 million) for the six months ended June 2012. The cash flows related to investing activities primarily stem from investments in, and maturities of, short-term bank deposits during the respective periods.
Net cash provided by financing activities for the six months ended June 30, 2013 was NIS 46.0 million ($12.7 million), compared to net cash provided by financing activities of NIS 52.3 million ($14.5 million) for the six months ended June 2012. The cash flows from financing activities primarily reflect the direct and private placements completed in February 2013 and 2012.
Conference Call and Webcast Information
BioLineRx will hold a conference call to discuss its second quarter 2013 results today, August 6, 2013, at 10:00 a.m. EDT. To access the conference call, please dial 1-888-668-9141 from the U.S. or +972-3-918-0609 internationally. The call will also be available via live webcast through BioLineRx’s website. A replay of the conference call will be available approximately two hours after completion of the live conference call. To access the replay, please dial 1-888-782-4291 from the U.S. or +972-3-925-5927 internationally. The replay will be available through August 9, 2013.
(Tables follow)
About BioLineRx
BioLineRx is a publicly-traded biopharmaceutical development company. BioLineRx is dedicated to building a portfolio of products for unmet medical needs or with advantages over currently available therapies. BioLineRx’s current portfolio consists of seven clinical stage candidates: BL-1040, for prevention of pathological cardiac remodeling following a myocardial infarction, and which has been out-licensed to Ikaria Inc., is currently undergoing a pivotal CE-Mark registration trial; BL-5010 for non-surgical removal of skin lesions has completed a Phase 1/2 study; BL-8040 for treating acute myeloid leukemia (AML) and other hematological cancers has commenced a Phase 2 study; BL-7040 for treating inflammatory bowel disease (IBD) has completed a Phase 2a trial; BL-8020 for hepatitis C (HCV) has commenced a Phase 1/2 study; BL-1021 for neuropathic pain is in Phase 1 development; and BL-1020 for schizophrenia. In addition, BioLineRx has four products in various pre-clinical development stages for a variety of indications, including central nervous system diseases, infectious diseases, cardiovascular and autoimmune diseases.
BioLineRx’s business model is based on acquiring molecules mainly from biotechnological incubators and academic institutions. The Company performs feasibility assessment studies and development through pre-clinical and clinical stages, with partial funding from the Israeli Government’s Office of the Chief Scientist (OCS). The final stage includes partnering with medium and large pharmaceutical companies for advanced clinical development (Phase 3) and commercialization. For more information on BioLineRx, please visit www.biolinerx.com, the content of which does not form a part of this press release.
Various statements in this release concerning BioLineRx’s future expectations constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include words such as “may,” “expects,” “anticipates,” “believes,” and “intends,” and describe opinions about future events. These forward-looking statements involve known and unknown risks and uncertainties that may cause the actual results, performance or achievements of BioLineRx to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Some of these risks are: changes in relationships with collaborators; the impact of competitive products and technological changes; risks relating to the development of new products; and the ability to implement technological improvements. These and other factors are more fully discussed in the “Risk Factors” section of BioLineRx’s most recent annual report on Form 20-F filed with the Securities and Exchange Commission on March 12, 2013. In addition, any forward-looking statements represent BioLineRx’s views only as of the date of this release and should not be relied upon as representing its views as of any subsequent date. BioLineRx does not assume any obligation to update any forward-looking statements unless required by law.
Contact:
Garth Russell / Todd Fromer
KCSA Strategic Communications
1 212-896-1250 / 1 212-896-1215
grussell@kcsa.com / tfromer@kcsa.com
Tsipi Haitovsky
Public Relations
+972-3-6240871
tsipih@netvision.net.il